Technologies
Solutions for Surgeons
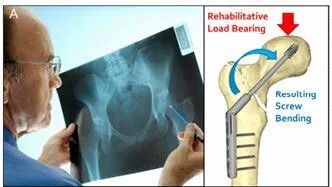
Figure 1. A) After hip-fracture diagnosis, a DHS is used to treat a patient’s fracture. B) Schematic showing how screw bending causes the screw body to move relative to a straight pin attached to the back of the screw: (i) unloaded; (ii) bending under load. The screw contains a radiopaque tungsten pin which threads into the dynamic hip screw using existing screw threads. C) Prototype device radiographically imaged within a cadaver. As in (B), the rod moves as force is applied to the femur upon standing.
Our goal is to develop sensors to non-invasively detect and monitor diseases to inform clinical decisions and outcomes using radiographs already acquired as standard of care.
We have solutions for:
Spinal Fusion
Hip and Long Bone Fractures
Trauma Plates
Detecting Infection
These devices and methods are designed to non-invasively track fracture healing and help determine when patients can bear weight, or if additional interventions are needed to accelerate healing and reduce complications. Here, we will focus on the market for sliding hip screws and cephalomedullary nails, used to treat intertrochanteric hip fractures (Figure 1). Specifically, our sensor is a passive metal device that attaches to sliding hip screws using existing threaded holes. Fracture stability could be easily measured inhuman cadavers and readings agreed with computer simulations. A pre-pilot study in a sheep demonstrated that the device could track fracture healing in vivo.
Medical Significance
Figure 1. A) After hip-fracture diagnosis, a DHS is used to treat a patient’s fracture.
Each year in the United States, over 300,000 hip fractures require surgical stabilization, which involves placing a sliding hip screw (SHS) through the fractured neck and into the femoral head (Figure 1A).
Initially, the SHS experiences a significant bending moment upon weight-bearing, which lessens over time as the fracture callus proliferates and strengthens. However, there exists a clinical gap in the current cycle of care. In the presence of delayed healing, which is a common occurrence among an elderly patient population, the hardware and bone will fatigue and fail. Such a failure requires costly revision surgery that carries a significant risk of morbidity and mortality. The average hip fixation hardware failure rate is around 5%; however, this can increase to over 20% in elderly patients with comorbidities such as osteoporosis, unstable fractures, and poor initial fracture reduction. Indeed, because these patients are frail, the 1-year all-cause mortality rate for patients readmitted within 30 days of surgery is 56%, compared to 19% for those not readmitted. In addition to a heightened mortality rate, an implant failure carries a significant increase in overall cost-of-care, increasing the $47,000 average cost by as much as 300%. Despite the consequences of fracture nonunion and eventual implant failure, the current postoperative cycle of care does not provide clinicians with the information they need to assess their patient’s risk of catastrophic implant failure and intervene if needed. In summary, this and similar devices can help physicians manage and monitor hip fractures more precisely, and detect non-delayed union and intervene earlier to prevent further pain, disability, and reoperations.